what are hydrocarbons class 10 Organic class chemistry hydrocarbons icse solutions questions short plus types aplustopper
Organic chemistry is a fascinating and complex field of study, and one aspect of it that truly captures my interest is hydrocarbons. If you’re unfamiliar with hydrocarbons, they are simply molecules composed entirely of hydrogen and carbon atoms. Despite the simplicity of their composition, hydrocarbons can take on a variety of forms, each with its own unique properties and characteristics. One way to classify hydrocarbons is by their structure. There are two main types of hydrocarbon structures: aliphatic and aromatic. Aliphatic hydrocarbons have straight or branched chains of carbon atoms, while aromatic hydrocarbons have a ring-like structure. Within each of these categories, there are several subcategories based on the number and arrangement of carbon atoms. Another way to classify hydrocarbons is by the type of bonds between their carbon atoms. Hydrocarbons can have single, double, or triple bonds, and each type of bond affects the molecule’s properties. For example, hydrocarbons with double bonds tend to be more reactive than those with single bonds. One of the most common and important types of hydrocarbons is methane. Methane is the simplest hydrocarbon, with just one carbon atom and four hydrogen atoms. It is a colorless, odorless gas that occurs naturally in the atmosphere, as well as in fossil fuels. Another important group of hydrocarbons is the alkanes. Alkanes are saturated hydrocarbons, meaning they have only single bonds between their carbon atoms. They are commonly used as fuels due to their high energy content and low reactivity. A third group of hydrocarbons is the alkenes. Alkenes are unsaturated hydrocarbons, meaning they have at least one double bond between their carbon atoms. They have a variety of uses, including as building blocks for plastics and other materials. Finally, there are the aromatic hydrocarbons, which are characterized by their ring structure. Perhaps the best-known example of an aromatic hydrocarbon is benzene, which has a six-carbon ring with alternating double bonds. Benzene is a highly reactive and toxic substance, but it is also a key building block for many important chemicals and materials. In conclusion, hydrocarbons are a fascinating and diverse group of molecules with a wide range of applications. Whether you are studying chemistry as part of your academic pursuits or simply have an interest in the world around you, understanding the basics of hydrocarbons can give you a deeper appreciation for the complexity and beauty of the natural world.
If you are looking for Classification of hydrocarbons Carbon and its Compounds-Science - Class 10 you’ve came to the right web. We have 5 Images about Classification of hydrocarbons Carbon and its Compounds-Science - Class 10 like ICSE Solutions for Class 10 Chemistry - Organic Chemistry - A Plus Topper, [23+] 1naming Hydrocarbons Chemistry If8766 Pg 96 Answers, 9.2 and also ICSE Solutions for Class 10 Chemistry - Organic Chemistry - A Plus Topper. Read more:
Classification Of Hydrocarbons Carbon And Its Compounds-Science - Class 10
 www.nextgurukul.inhydrocarbons
www.nextgurukul.inhydrocarbons
Classification Of Hydrocarbons
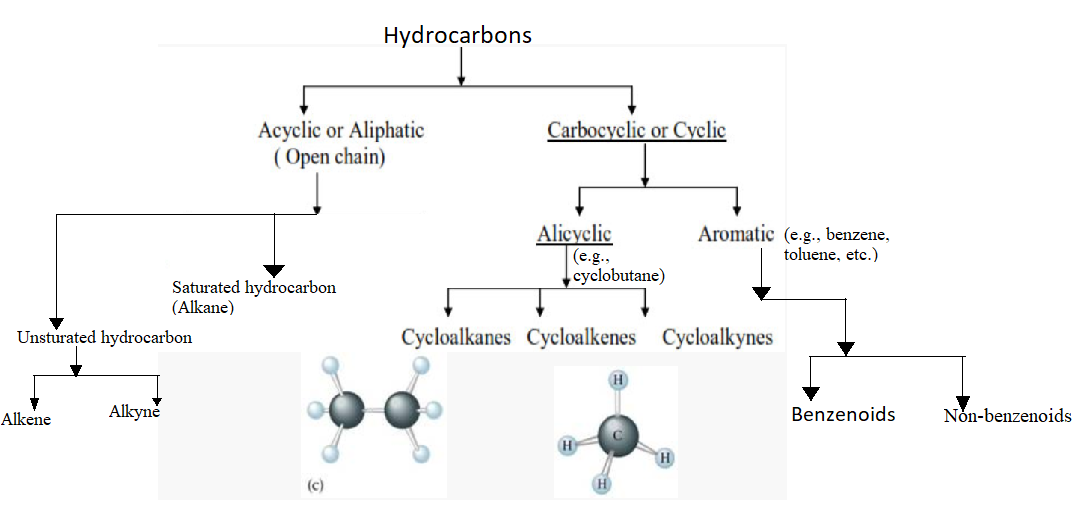 selfstudypoint.inhydrocarbons
selfstudypoint.inhydrocarbons
[23+] 1naming Hydrocarbons Chemistry If8766 Pg 96 Answers, 9.2
![[23+] 1naming Hydrocarbons Chemistry If8766 Pg 96 Answers, 9.2](https://i.pinimg.com/originals/5a/2a/b9/5a2ab9fef36b6d9792a1f0750579b0ca.png) heavy948.blogspot.comUnsaturated Hydrocarbons:
heavy948.blogspot.comUnsaturated Hydrocarbons:
 www.nextgurukul.inICSE Solutions For Class 10 Chemistry - Organic Chemistry - A Plus Topper
www.nextgurukul.inICSE Solutions For Class 10 Chemistry - Organic Chemistry - A Plus Topper
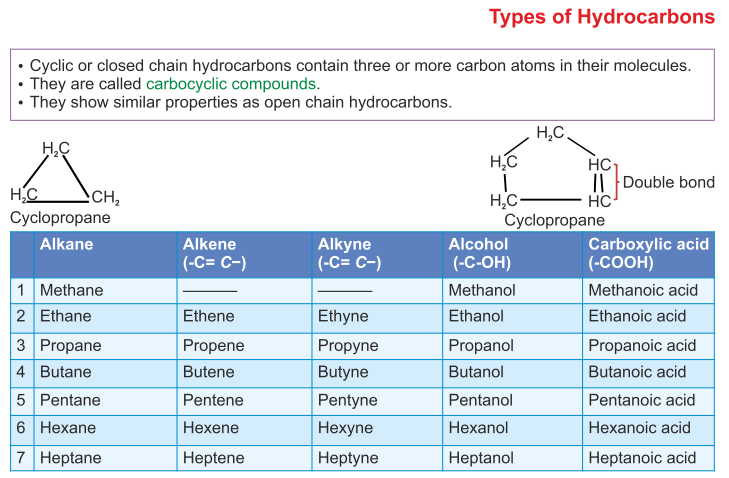 www.aplustopper.comorganic class chemistry hydrocarbons icse solutions questions short plus types aplustopper
www.aplustopper.comorganic class chemistry hydrocarbons icse solutions questions short plus types aplustopper
Unsaturated hydrocarbons:. Classification of hydrocarbons. Classification of hydrocarbons carbon and its compounds-science